
The groups are actually numbered up at the top of the table. The periodic table has all sorts of cool information just based on its layout.Ī column on the periodic table is known as a group or family. Later, you will find out that those row numbers will match perfectly with the principle quantum number \(n\) from atomic theory. You might look and think "wait, I counted nine", and that would be technically wrong because those bottom two rows with elements 58-71, and 90-103 are actually from rows (periods!) 6 and 7 from the main table above them. There are seven periods on the periodic table. Periods and GroupsĪ row on the periodic table is called a period.
#Complete periodic table chemistry pdf#
Here's a nice Periodic Table and more pdf for you to use for this class. The periodic table is your ultimate conversion chart for converting any substance into another substance and doing so with exact proper amounts (masses and moles). Knowing how numbers work and how ratios work is KEY to understanding and working chemistry stoichiometry problems. You'll only have to go to grams IF the number of moles is asked for. Just work the problem in pounds - it will work. there is really no reason to convert to grams first and then back out to pounds later. How? Well IF the problem is stated in say pounds, and then wants the answer in pounds. Quite the rigorous path for "all" problems. And finally, convert those grams into any other unit needed that might be asked for. Then (if need be) convert your answer in moles into grams. Then, convert those grams in to moles and work the problem in moles only. In general, to work all types of stoichiometry problems, we say to convert all masses to grams first.

Those atomic weights are the number of grams you will need of that element in order to have exactly 1 mole of that element. Counting by number is the molar amount, while measuring by mass is the. This helps tremendously when having to convert from moles to mass as we often do in chemistry. Because of that old definition, we were able to say that all those atomic weights are in grams per mole of substance or abbreviated g/mol. So why DO we seem to concentrate on the "gram" as our go to guy on the periodic table for atomic weights and ultimately for molar masses and molecular weights? Well the key here is the way we historically defined the mole. All chemical ratios work just as well with masses as they do with our oh so familiar moles. You can work chemistry mass problems in any mass you want and it will still work because the masses are relative to each other. Not to mention the myriad of masses represented by all the metric prefixes to prepend to "gram". short tons, long tons, drams, grains, or stones. You could think in pounds, or kilograms, or ounces, or even tons, or heaven forbid. Relative masses means that they are all corrected relative to each other. BUT it would be much much better for you to realize that those could be ANY unit of weight/mass you choose and the whole table would still be correct.
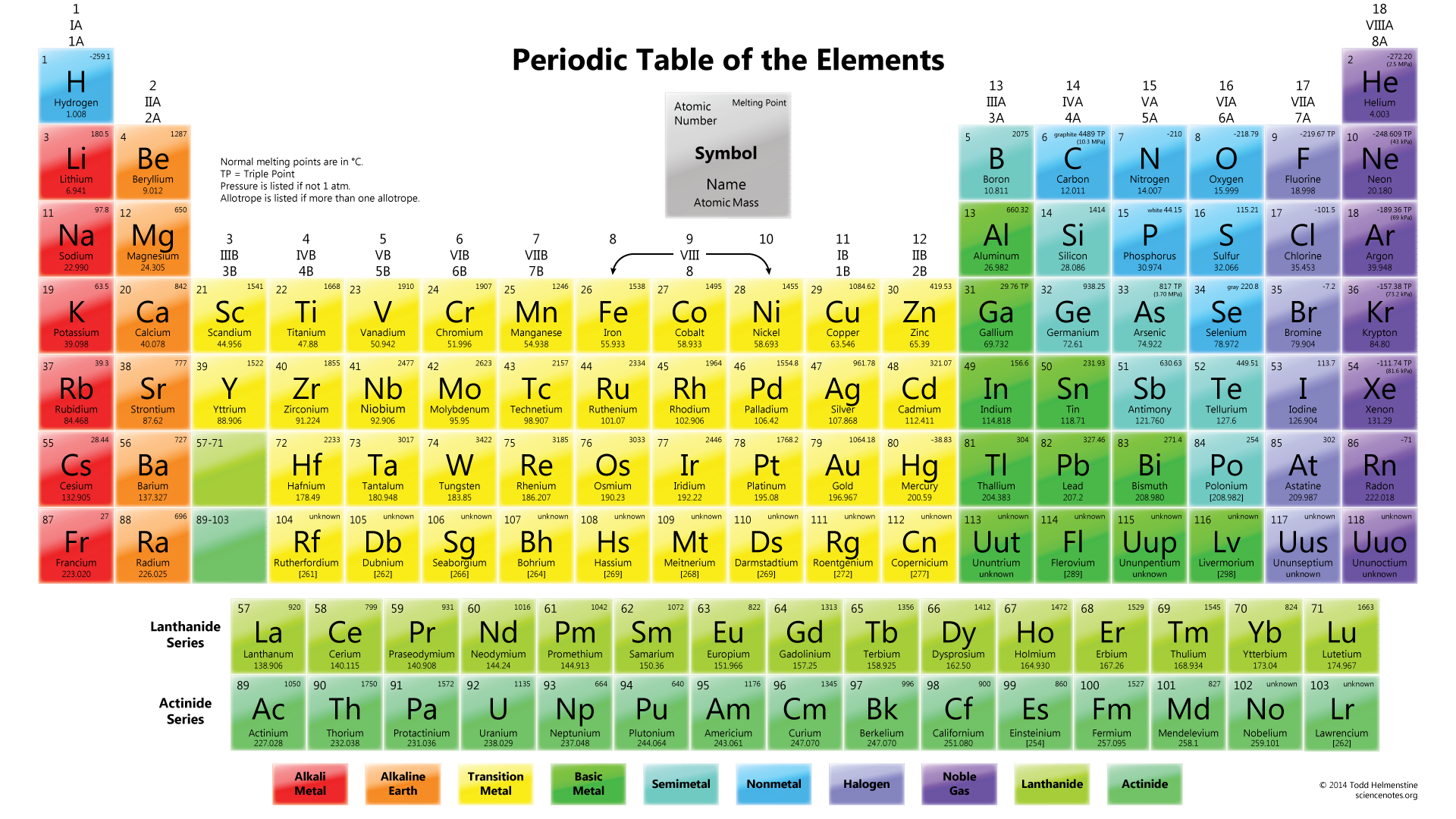
"Well, I know the weights are in grams because that is how I learned it in high school". Notice how the atomic weights have no units after them. Hey you! LOOK again at any periodic table - including the one above. The diagram below illustrates the parts and their definitions. Make sure that you know what each of these parts is and what it represents. *Note: If you click on the table, you'll launch it into its own window/page on your browser. These three pieces of data are the elemental symbol, the atomic number (typically given the symbol, Z, and the atomic weight. In it's simplest form (shown below), each entry only has three pieces of information that you will need to know.

The periodic table can often be presented with an abundance of data about each and every element listed.


 0 kommentar(er)
0 kommentar(er)
